8 years have passed since revolutionary changes in Korean Pharmaceutical industry. In the US, over-the-counter (OTC) drugs have long been available in non-pharmacy outlets, and in the UK, general sale list medicines can be supplied without the need for the supervision of a pharmacist. Recently, the Japanese government allowed medications for colds or fever and painkillers, which account for 95% of general pharmaceuticals, to be sold at retail stores through the amendment to the Pharmaceutical Affairs Act. Among the key trends of the global OTC market are major changes in product distribution. However, in Korea, the independent pharmacy model traditionally has been the method of OTC distribution for many years. 19 years have passed since the first civil petition for non-pharmacy distribution of selected OTC drugs. At last in 2012, Korea revised the Pharmaceutical Affairs Act, allowing selected non-prescription drugs to be distributed by non-pharmacy outlets. The revised Pharmaceutical Affairs Act restricted the number of selected OTC drugs to under 20. The new Act included the registrations of non-pharmacy outlets and the mandatory education of the sellers. We must discuss the criteria for placement of medicines on the GSL,
 Nutrition and vitamins. Internet shopping mall is rising .
Nutrition and vitamins. Internet shopping mall is rising .
the reclassification system based on the safety profile of the medicines, and the expansion of non-pharmacy outlets. In preparation for the aged society, we should implement health policies to lower the price of pharmaceuticals by adopting the market principle thus giving consumers a broader choice
However, still many things need. Low level of understanding on medical law combined by vulnerability of store.
By Duke DM Kwon. Senior Editor
Copyright (c ) Korean Women's Health News. All rights reserved.
 유전자조작기술 (CRISPR)
현재 유전자 조작 기술은 CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) 기술이 주도하고 있습니다. 이 기술은 특정 유전자를 정확하게 수정하는 강력한 도구로, 미국의 여러 기업들이 이 분야에서 선두를 달리고 있습니다.여기 몇몇 주목할만한 CRISPR 기업들이 있습니다:Verve Therapeutics: Cambridge, Massachusetts에 위치한 Verve Therapeutics는 심혈...
유전자조작기술 (CRISPR)
현재 유전자 조작 기술은 CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) 기술이 주도하고 있습니다. 이 기술은 특정 유전자를 정확하게 수정하는 강력한 도구로, 미국의 여러 기업들이 이 분야에서 선두를 달리고 있습니다.여기 몇몇 주목할만한 CRISPR 기업들이 있습니다:Verve Therapeutics: Cambridge, Massachusetts에 위치한 Verve Therapeutics는 심혈...
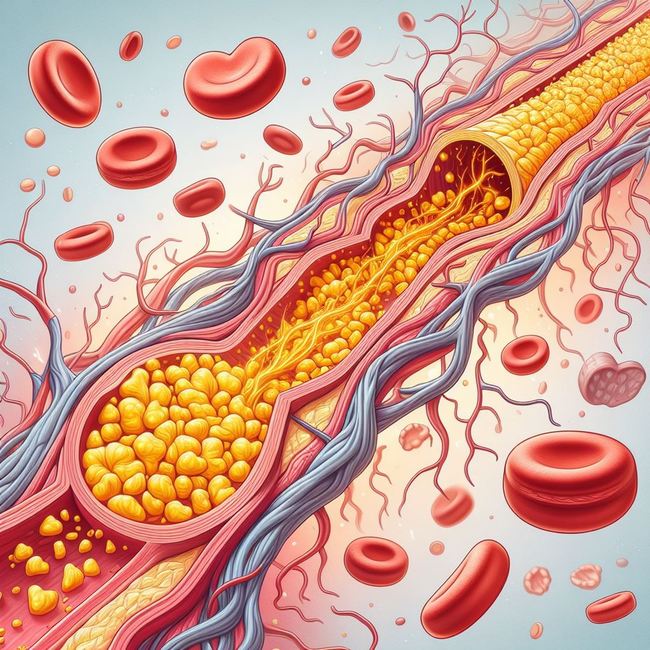
 스타틴계열 약물이 뭐예요?
흔히 자주 듣는 의학전문 용어 중에 스타틴계열 약물이라는 얘기를 자주 들어 보셨을 겁니다. 약물의 주요 성분의 이름이 ~스타틴 이라고 끝나는 데 따라 붙은 이름인데요. 주로 심혈관계에 작용하는 약물들이 포함됩니다.리피토나 조코와 같은 세계적인 베스트셀러 약품도 이런 스타틴 계열 약물중의 하나입니다.정보가 도움이 되신다면, ...
스타틴계열 약물이 뭐예요?
흔히 자주 듣는 의학전문 용어 중에 스타틴계열 약물이라는 얘기를 자주 들어 보셨을 겁니다. 약물의 주요 성분의 이름이 ~스타틴 이라고 끝나는 데 따라 붙은 이름인데요. 주로 심혈관계에 작용하는 약물들이 포함됩니다.리피토나 조코와 같은 세계적인 베스트셀러 약품도 이런 스타틴 계열 약물중의 하나입니다.정보가 도움이 되신다면, ...

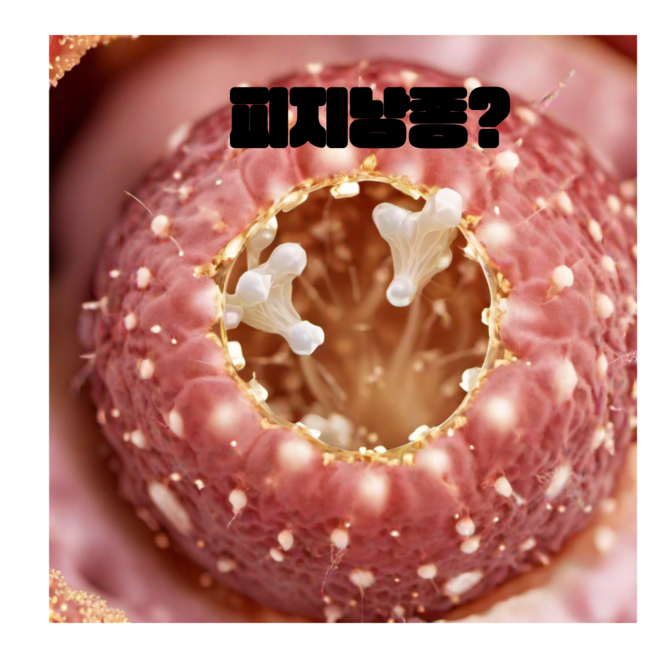 피지낭종 - 말랑말랑할 줄 알았다면 이 영상을 잘 보세요
피지낭종 - 말랑말랑할 줄 알았다면 이 영상을 잘 보세요
 교통혁명과 로봇으로 여가시간이 늘어난 다면.
교통혁명과 로봇으로 여가시간이 늘어난 다면.
 서울대 출신 약사가 백신 대신 빵을 만드는이유
서울대 출신 약사가 백신 대신 빵을 만드는이유
 지방소멸 방지의 일환으로 일자리와 문화공간을
지방소멸 방지의 일환으로 일자리와 문화공간을